Describe the Properties of Alkali Metals
This is because all the atoms of alkali metals have one valence electron. 1610 assess and manage risks associated with the storage and use of alkali metals and recall that alkali metals are easily cut are shiny when freshly cut and tarnish rapidly in air.
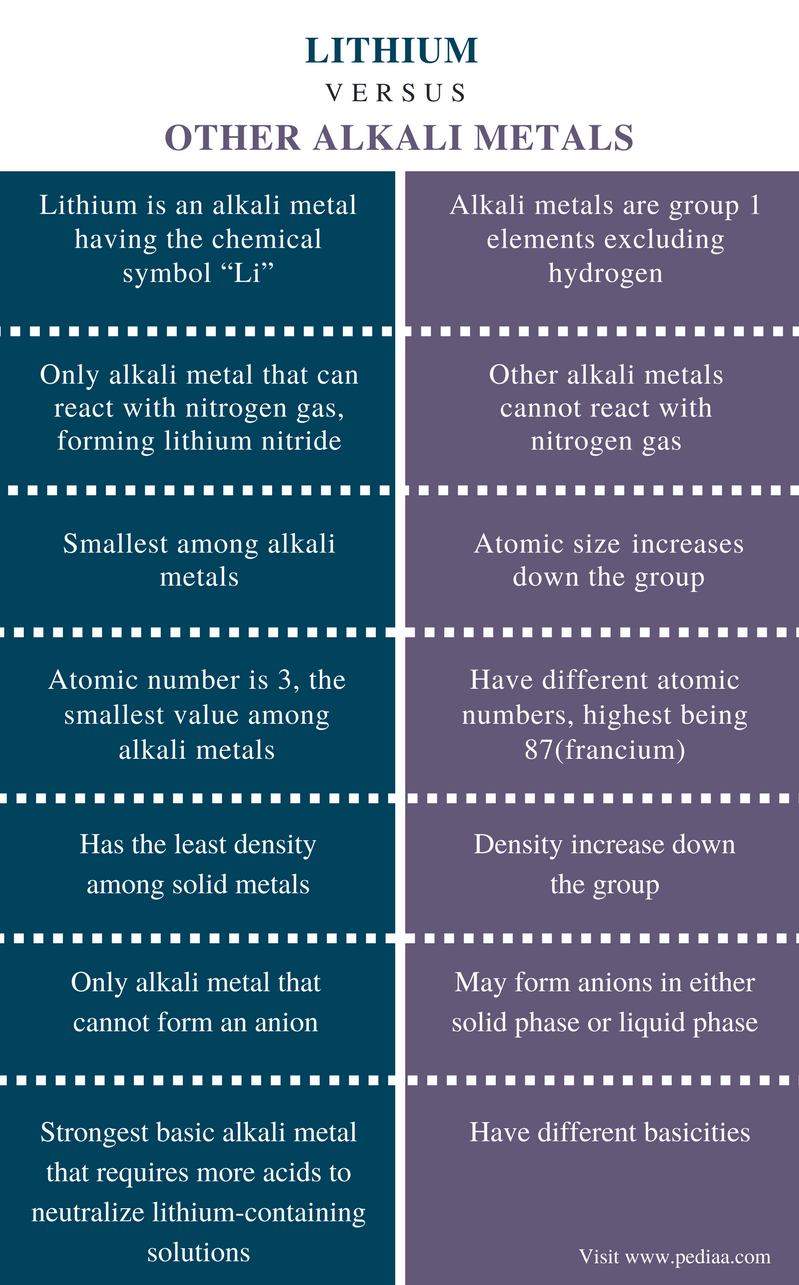
Difference Between Lithium And Other Alkali Metals Definition Chemical Facts Properties Differences
The alkali metals are highly electropositive which means they readily lose.
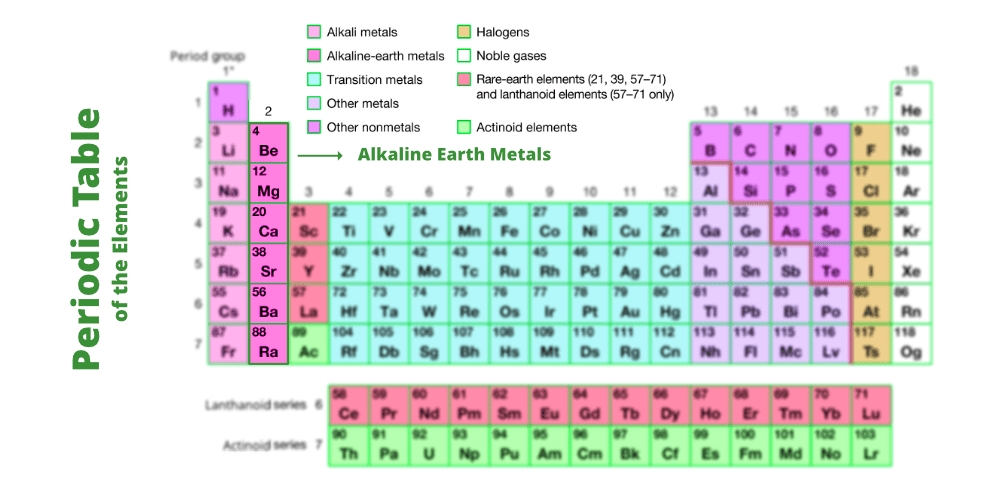
. Based on their electronic arrangement explain whether they exist alone in nature. Based on their electronic arrangement explain whether they exist alone in nature. 169 demonstrate knowledge and understanding that the alkali metals have low density and the first three are less dense than water.
All the alkali and alkaline earth metals are silvery white in appearance. Alkali metals have one electron in their outer shell which is loosely bound. Summary of Common Alkaline Earth Properties Two electrons in the outer shell and a full outer electron s shell Low electron affinities Low electronegativities Relatively low densities Relatively low melting points and boiling points as far as metals are concerned Typically malleable and ductile.
All alkali metals are highly reactive towards the more electronegative elements such as oxygen and halogens. They include Sodium Lithium Potassium etc. In its chemical reactivity lithium more closely resembles Group 2 IIa of the periodic table than it does the other metals of its own groupIt is less reactive than the other alkali metals with water oxygen and.
They are highly reactive metals They have low electro negativity They have low ionization energy They dont exist alone in nature They have low densities. 10 rows Why are Melting and Boiling Points of Alkali Metals Low. Since the alkali metals are the most electropositive the least electronegative of elements they react with a great variety of nonmetals.
Describe Chlorines physical properties. Chemical properties of alkali metals. Some characteristic chemical properties of alkali metals are described blow.
Alkali metals are the elements in group 1 of the periodic table. The alkali metals exhibit many of the physical properties common to metals although their densities are lower than those of other metals. Due to low ionization power they are highly.
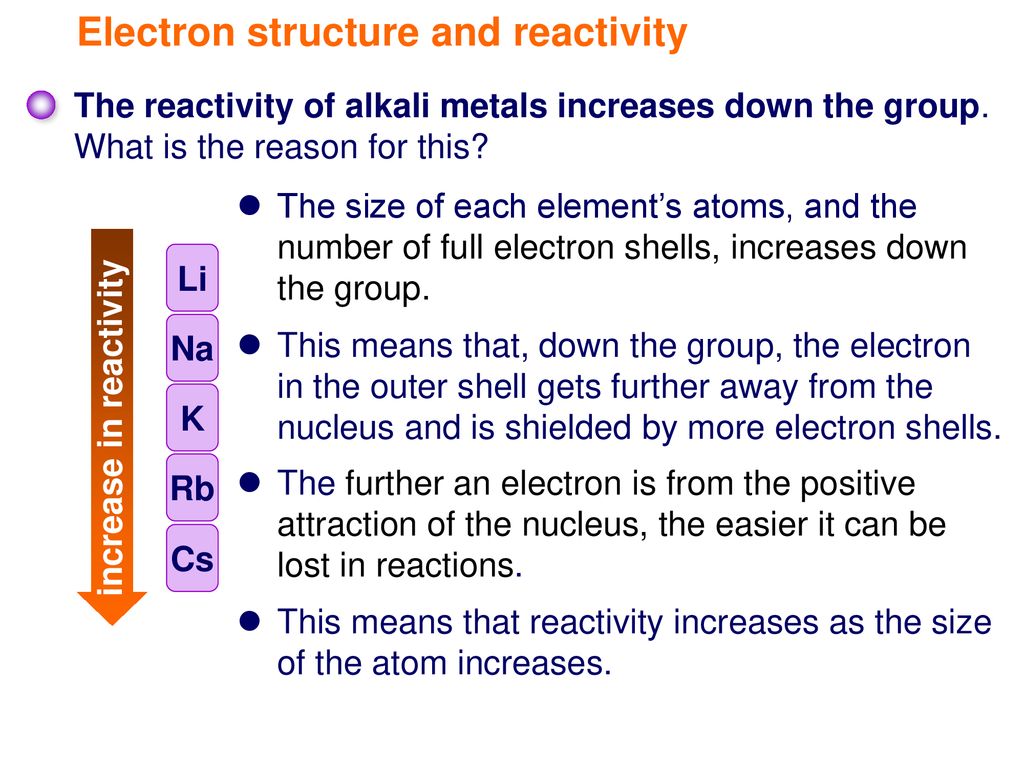
GreenYellow can burn skin in. Describe Chlorines chemical properties. The reactivity of alkali metals increases from Li to Cs since the ionisation energy decreases down the group.
Due to their large atomic size they have low density. The alkali metals have the silver-like lustre high ductility and excellent conductivity of electricity and heat generally associated with metals. Chemistry 22062019 1200 KKHeffner02.
The alkali metals are s-block elements that is their valence electrons are located in the s orbital which can hold a maximum of 2 electrons and are therefore very reactive. 1 The alkali metals are the elements found in the group 1 of the periodic table. Alkali metals have general electronic configuration ns1 E Alkali metals exhibit on oxidation state of1.
Are soft they can be cut with a knife have relatively low melting points. Describe the properties of alkali metals. The alkali metals have low melting points ranging from a high of 179 C 354 F for lithium to a low of 285 C 833 F for cesium.
Although alkali metals exhibit similar chemical properties they differ in reactivity. 1 question Describe the properties of alkali metals. The metallic property of alkali metals increases from lithium to caesium.
An alkali metal can easily lose its valence electron to form the univalent cation. The reactivity of alkali metals increases when going down Group 1. All alkali metals are highly reactive with water halogens and acids.
Alkali metals have low electronegativities. They dont react as violently Some dont react at all. Their low ionization energies result in their metallic properties and high reactivities.
Alkali metals are the most electropositive elements while alkaline earth metals are comparatively less. The middle of the table left of the staircase. Being very soft alkali metals have low.
Low melting and boiling point. Both alkali and alkaline earth metals are soft however alkaline earth metals are harder than the alkali metals. Alkali metals have low ionization potential.
Which statement best explains the relationship between an area is geography and the temperature of its surface water. Lithium is the lightest metallic element. What are the chemical properties of alkali earth metals.
This gives them the largest atomic radii of the elements in their respective periods. They react with water to produce an alkaline metal hydroxide solution and hydrogen. Alkali metals exhibit high chemical reactivity.
Alkali metals have one electron in their outer shell which is loosely bound. All the alkaline earth metals found are present in nature. Group 1 - the alkali metals The group 1 elements are all soft reactive metals with low melting points.
Binds with sodium highly reactive. Alkali metals are very reactive. 6 rows Chemical Properties.
Describe the properties of alkali metals. Where are transition metals located. Atomic radius increases down the group from lithium to caesium.
Low melting and boiling points. 1 Get Other questions on the subject. All alkali metal exhibit similar chemical properties.
Based on their electronic arrangement explain whether they exist alone in nature.

Alkali Metal Definition Properties Facts Britannica

Chemistry Alkali Metals By Ashfordschool Issuu
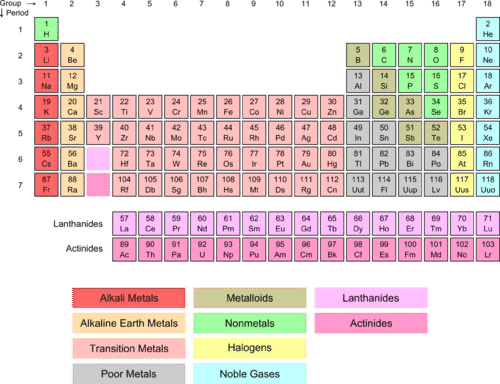
6 9 Hydrogen And Alkali Metals Chemistry Libretexts

General Characteristics Of Compounds Of Alkali Metals Emedicalprep
Difference Between Alkali Metals And Alkaline Earth Metals Definition Properties Examples

Who Are The Alkali Metals List The 6 Members Of The Alkali Metals Ppt Download
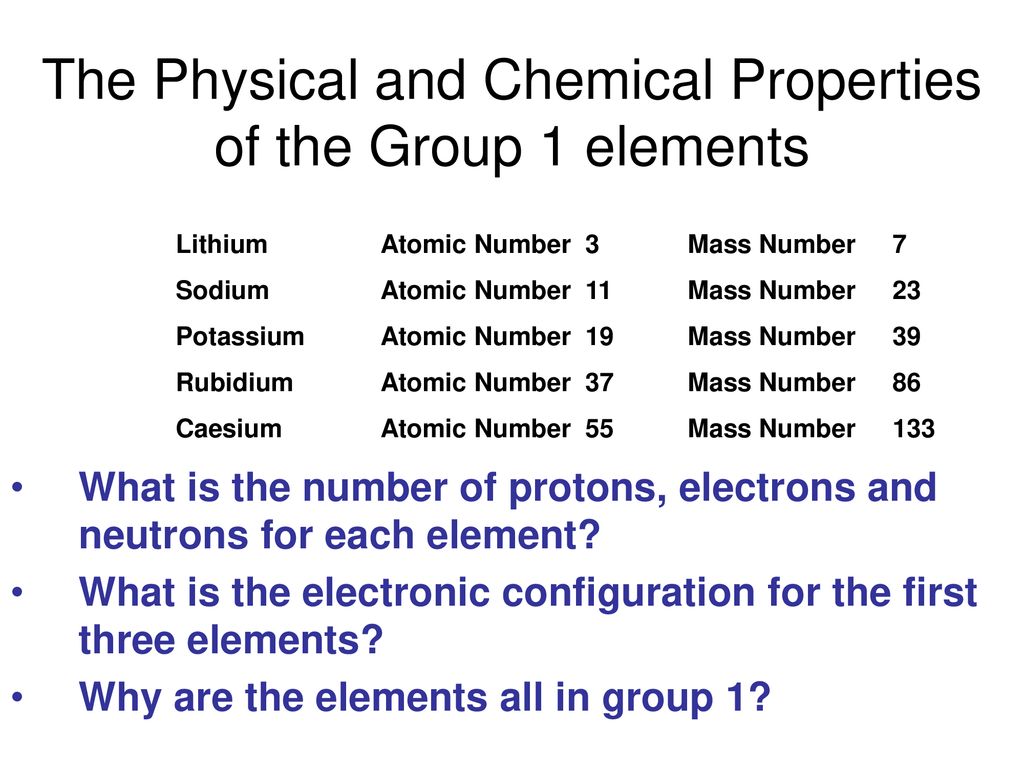
The Physical And Chemical Properties Of The Group 1 Elements Ppt Download

Who Are The Alkali Metals List The 6 Members Of The Alkali Metals Ppt Download

Alkali Metals Properties Group Examples What Are Alkali Metals Video Lesson Transcript Study Com

Chemistry Alkali Metals Ppt Download
Chemistry Alkali Metals Ppt Download

How The Periodic Table Groups The Elements Live Science

Characteristics Of The Compounds Of Alkaline Earth Metals Geeksforgeeks

General Characteristics Of Compounds Of Alkali Metals Emedicalprep
What Are The Similarities Between Alkali Metals And Alkaline Earth Metals Quora

Elements Of S Block Properties Of The First Group Elements 1a Alkali Metals In The Periodic Table Science Online

Alkali Metal Definition Location In Periodic Table Properties

The Alkali Metals Prezentaciya Onlajn
Alkali Metals Properties Electronic Configuration Periodic Trends Uses
Comments
Post a Comment